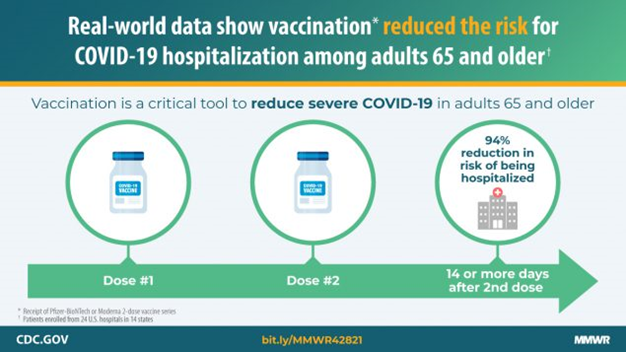
Clinical trials suggest high efficacy for COVID-19 vaccines, but evaluation of vaccine effectiveness against severe outcomes in real-world settings and in populations at high risk, including older adults, is needed. In a multistate network of U.S. hospitals during January-March 2021, receipt of Pfizer BioNTech or Moderna COVID-19 vaccines was 94% effective against COVID-19 hospitalization among fully vaccinated adults and 64% effective among partially vaccinated adults aged ≥65 years old.
Monitoring the effectiveness of SARS-CoV-2 vaccination under routine public health use and specifically against severe outcomes in patients at higher risk, including older adults, is a high priority. These findings are consistent with efficacy determined from clinical trials in the subgroup of adults aged ≥65 years old. In the current report, Pfizer-BioNTech and Moderna vaccine products were equally represented, and approximately one half of the patients were aged ≥75 years, providing evidence of real-world effectiveness of both vaccines against an important measure of severe COVID-19 in older adults.
In assessing the impact of receiving only a single dose, no significant vaccine effectiveness <14 days after the first dose of a SARS-CoV-2 vaccine was detected. This suggests that bias is unlikely in the primary estimates of vaccine effectiveness from partial and full vaccination. If you haven’t already been vaccinated please consider it, these statistics are spot on, exactly what the initial trials revealed. Stay well, mask up indoors and stay tuned!